Abstract
Introduction: Delay in recovery or engraftment of neutrophils is a risk for infection and associated with poor prognosis after cancer chemotherapy or hematopoietic stem cell transplantation (SCT). Recent studies demonstrate that intestinal microbiota plays a critical role in the development of bone marrow (BM) granulopoiesis in infants after birth (Deshmukh, et al. Nat Med 2014). Here, we addressed the impact of microbiota on neutrophil recovery after chemotherapy and engraftment after syngeneic SCT.
Method: Wild type (WT) or RAG1-/- B6 mice were given a combination of ampicillin 1g/L, streptomycin 1g/L, and vancomycin 1g/L in drinking water daily from day -7 or left untreated.Recipient WT, RAG1-/-, or IL-17A-/- B6 mice were lethally irradiated and transplanted with 7,500 purified lineage-sca-1+c-kit+ cells plus 20,000 granulocyte-macrophage progenitors (GMPs) purified from congenic B6-CD45.1 mice on day 0. For in vivo T cell depletion, mice were i.p. injected with 200 μg of anti-CD90 monoclonal antibodies (αCD90) thrice a week from day -7. To assess reactive granulopoiesis after chemotherapy, B6 mice were i.p. injected with 5-fluorouracil (5-FU) at a dose of 200 mg/kg.
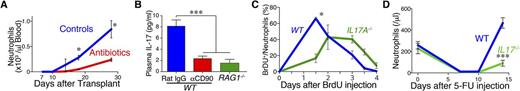
Results: To address if gut microbiota could play a role in neutrophil engraftment, gut decontamination using the antibiotics was performed from day -7 of SCT. We found that gut decontamination significantly delayed engraftment of neutrophils, suggesting that microbiota promotes neutrophil engraftment after SCT (Figure A). Plasma levels of G-CSF and a G-CSF-inducing cytokine, IL-17A were significantly elevated 14 days after SCT, while the antibiotics completely abrogated upregulation of these cytokines. IL-17A expression in host αβ and γδ T cells was significantly suppressed by the antibiotic treatment after SCT, suggesting that microbiota stimulates host T cells to produce IL-17A after SCT. Next, we studied the role of IL-17A in neutrophil engraftment using IL-17A-/- mice as recipients. IL-17A deficiency phenocopied the antibiotic treatment, with lower plasma levels of G-CSF and delayed neutrophil engraftment compared to WT recipients. When recipients were devoid of host T cells by administration of αCD90 mAbs or by using RAG1-/- mice as recipients, plasma levels of both IL-17A and G-CSF were significantly decreased, suggesting a critical role of host T cells in IL-17A secretion in response to microbiota (Figure B and data not shown). Neutrophil engraftment was delayed in RAG 1 -/- mice, while gut decontamination did not impact on neutrophil engraftment in RAG 1 -/- recipients, ruling out direct toxicity of the antibiotics on engraftment and further emphasizing a critical role of host T cells in granulopoiesis after SCT. Flow cytometric analysis demonstrated that the numbers of GMPs in the BM of IL17A-/- recipients were significantly less than those in WT controls, suggesting that IL-17A promoted granulopoiesis in the BM. Emergence of BrdU+ neutrophils in the peripheral blood after BrdU injection on day +18 after SCT was delayed in IL17A-/- recipients compare to WT controls, indicating that IL-17A not only stimulates granulopoiesis but also facilitates neutrophil egress from the BM to peripheral blood (Figure C). Finally, we found that neutrophil recovery after chemotherapy with 5-FU was delayed in the antibiotic-treated mice or IL-17A-/- mice, suggesting that neutrophil recovery after chemotherapy was also dependent on IL-17A secretion in response to microbiota (Figure D). Plasma levels of IL-17A and T-cell IL-17A expression were significantly lower in naïve MyD88-/- mice than those in WT mice, suggesting that IL-17A production in T cells were dependent on MyD88 signaling.
Conclusion: Our study uncovers a novel role of gut microbiota in neutrophil engraftment after SCT and neutrophil recovery after chemotherapy; gut microbiota enhances granulopoiesis and neutrophil egress from the BM by stimulating T cells to produce IL-17A.
Figure: (A) Gut decontamination with antibiotics delayed neutrophil engraftment after SCT. (B) T cells were responsible to IL-17 production after SCT. (C) Neutrophil egress from BM was impaired in IL17A-/- recipients. (D) IL-17 played a critical role in reactive granulopoiesis after chemotherapy.
Teshima: Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Research Funding; Kyowa-Hakko Kirin: Research Funding; Pfeizer: Research Funding; Chugai: Research Funding; Bristol-Myers Squibb: Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal